COEs at Chemistry Innovation Research Centre for Bringing Speed and Quality to drug discovery.
Our Centre of Excellence is designed to support expanded discovery chemistry services where we cultivate expertise in emerging methodologies.
Discover how our Centre of Excellence unites various processes and expert insights in proven and emerging approaches.
Our Experts Leverage their Expertise in Proven and Emerging Methodologies

Centre of Excellence: Carbohydrate Chemistry
Jubilant Biosys has dedicated, highly qualified, and experienced synthesis and purification teams led by experienced project leaders to carry out SPPS and carbohydrate chemistry projects. The blend of experience and expertise leads to quick familiarization of project requirements, innovative strategies for synthetic route designing, and their execution.
The teams have considerable experience in the synthesis and purification from mg to a multi-gram scale of:
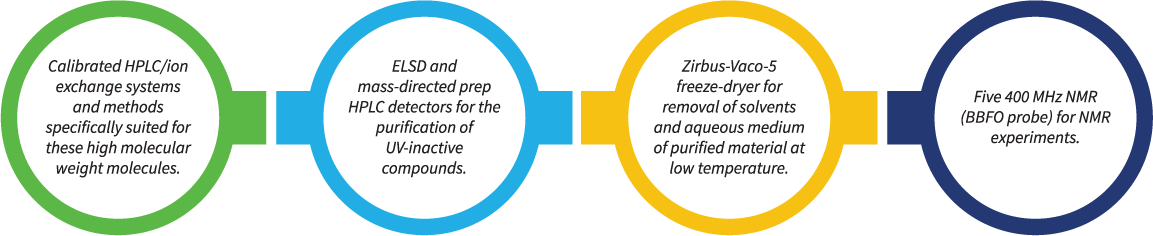
To support the synthetic teams, we have an equally experienced and dedicated analytical team equipped with advanced instruments like:
Over the past decade, our team of highly skilled FTEs has a proven track record in successfully delivering challenging carbohydrate chemistry projects.
Centre of Excellence: PROTAC, LYTAC & related Chemistry
Proteolysis targeting chimaeras (PROTACs), PHOTACS (photochemically targeted chimaeras) and LYTAC (lysozyme targeting chimaeras) are heterobifunctional molecules based on the combination of ubiquitin E3 ligase ligands with an inhibitor of the protein of interest (POI) via different types of linkers. With this design, the protein of interest can be degraded, which may have different biological effects compared to classical inhibition. This technique has some advantages, as it can be used for undruggable targets. Additionally, PROTACs act in very low concentrations, and they can provide increased selectivity for the target of interest rather than the original small molecule inhibitor.
Jubilant Biosys, keeping pace with the recent developments in the field of PROTAC, PHOTAC and LYTAC, created an efficient and highly skilled group of synthetic and analytical chemists led by project leaders trained from various national and international premier organizations to undertake the synthesis of these complex molecules. This group can synthesise these complex molecules in a very short time with very high purity. A variety of ligands (thalidomide, lenalidomide, pomalidomide, VHL, glycopeptides) with linkers of varying sizes, synthesised internally, is used regularly for the synthesis of these compounds.
The synthetic group is supported by a highly proficient analytical team, which purifies and analyses the intermediates and final compounds before shipping them to the clients. In the recent past, we have been able to synthesise, purify and characterise these molecules with molecular weights ranging from 450 to 2400. In addition to the synthesis of commercially available ligands on the multigram scale, we are also well-versed in developing novel structures.
We also have an elaborate Project management group which looks after the project from the initiation to delivery. This group also takes care of all the shipment requirements especially when required under controlled conditions (~ -20oC).
Centre of Excellence: Parallel & Library Synthesis
Parallel synthesis and Library synthesis are two important tools in modern drug discovery. They enable the synthesis of a large number of closely related analogues in a short time following general synthetic transformations. Thus, parallel library synthesis has the potential to reduce the cycle time of medicinal chemistry efforts significantly. Therefore, in the past few decades, we found that the synthesis of focused small molecule libraries are very useful for the optimization of drug leads.
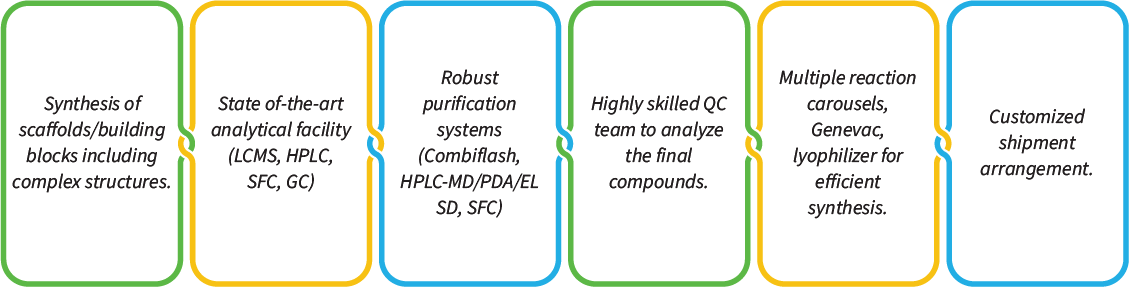
Jubilant Biosys has over 15 years of rich experience in the synthesis of custom libraries. We have a strong track record of delivering several focused libraries ranging from 20-300 compounds and large libraries of up to 6000 compounds. We have a dedicated team of highly skilled and experienced chemists capable of performing complex chemical synthesis and purification. We are fully equipped with state-of-the-art infrastructure and highly proficient in parallel synthesis, high throughput purification, compound analysis, and management.
Centre of Excellence: Lipid Chemistry
The demand for lipids in pharmaceutical applications is expected to rise due to their increasing use in drug development and many other pharmaceutical applications. Lipids are a highly influential component in the development of mRNA-based vaccines for COVID-19. Its massive demand in the development of COVID-19 vaccines for the global population has compelled manufacturers to ramp up their lipid production.
These are Different Types of Lipids Known in the Literature Based on their Properties

Our Capabilities:
Jubilant Biosys has over three years of rich experience in the synthesis of lipids with a dedicated team of 20-25 scientists.
Centre of Excellence: Solid State Chemistry
Jubilant Biosys offers Solid State Chemistry services to provide salt, co-crystal, and polymorph screening and selection activities to provide suitable crystallisation processes during all the development phases. Solid State Chemistry Division has a dedicated lab with state-of-the-art equipment and a team of experienced solid state chemists.
Benefits of Solid State Chemistry
Jubilant collaborates with pharmaceutical and biotechnology companies to advance their high-value intermediates and active pharmaceutical ingredients (APIs) from initial process development and scale-up through process validation and commercial manufacturing. APIs have a propensity to crystallise in different solid state forms. Understanding and knowing these forms can establish robust API and formulation processes, mitigate risks, minimise problems, and reduce development timelines.
Solid State Chemistry Requirements Throughout CMC Development
Since different polymorphs have different physicochemical properties, identifying a viable polymorph early during drug development is essential. By the end of Phase II, information about the solid state form must be provided to the FDA, including the propensity to exhibit polymorphism with other physicochemical data. JBL can help you through the drug development process by building knowledge about your solid state forms which ultimately can establish robust API/formulation processes, mitigate risks, minimise problems, and reduce development timelines.
Solid State Chemistry Services
Advanced Techniques and Characterisation Capabilities
Through the synergy between solid state chemistry and chemical development, our experienced scientists add great value to your pre-clinical project while decreasing timelines.
Overview
Our domain experts group has expertise in screening and selection, so they can skilfully guide you to make the right decisions at the right time.
Enabling Salt Screen
A drug candidate is at risk of failure due to poor oral bioavailability, handling issues or instability, or during the development of a new dosage form, a salt (or co-crystal) form will have the potential to dramatically improve the drug’s properties with a quick turnaround.
Polymorph Screening
Polymorph screening is designed to reduce the risks of drug development by finding the most stable form of the API and defining the form for clinical development and GMP manufacture. Our screening services are directed by highly trained chemists and analysts performing a scientific investigation tailored to your API properties, development phase and budget.
Salt and Co-Crystal Screening
The selection of an appropriate salt provides an opportunity to modify the API’s physicochemical properties and resultant biological characteristics. However, the salt form selected will influence a range of other properties. The screening for developable salt forms could strengthen the intellectual property of the API. A salt or co-crystal can also enable you to go over the next hurdle in the pharmaceutical drug development process, whether to move on to the initial toxicity studies or to go into clinical drug development.
Amorphous Solids
Amorphous drug substances can exhibit enhanced solubility over a crystalline form. However, the amorphous solid may be unstable to recrystallisation during the shelf life of a product unless it is stabilised as an amorphous solid dispersion. We can rapidly identify the most suitable polymers at the right concentration for early drug formulation development. The scope will be tailored to your needs, development phase and budget.
Crystallisation Process Development and Optimisation
Taking a quality-by-design approach, we offer knowledge-based crystallisation process development to achieve robust and scalable crystallisations that yield reproducible high-quality solids. A thorough understanding of the solid form accompanied by solubility data is the base of our method.
A fast and efficient knowledge transfer between our solid state and process development scientists assures a polished crystallisation process that is focused on the following aspects:
Centre of Excellence: Electrochemistry / Organic Synthesis / PhotoRedox Chemistry
Electrochemistry in Organic Synthesis
In recent years electro organic synthesis has emerged as a promising green methodology in organic chemistry and a quick alternative to conventional organic synthesis in academic labs.
Several breakthrough research papers using electrochemical reactions which are alternatives to Birch reduction by avoiding the use of Na/Li-metal, reduction of amide to an amine by avoiding the use of pyrophoric LAH or borane in a scalable manner, reduction of the double bond to single bond by avoiding Pd/C, epoxidation without the use of peroxide reagent, and chemoselective reduction of hetero arenes, have attracted chemical industries to adopt this new technology.
Other high potential reactions such as decarboxylative sp3-sp2 and sp3-sp3 cross-coupling, O-arylation of alcohols, sulfonylation of aryl halides, and dimerization of amine are successfully carried out using electrochemical reactions. All these reported new methods have drastically reduced the number of steps compared to conventional synthesis, reducing time and waste by improving yield.
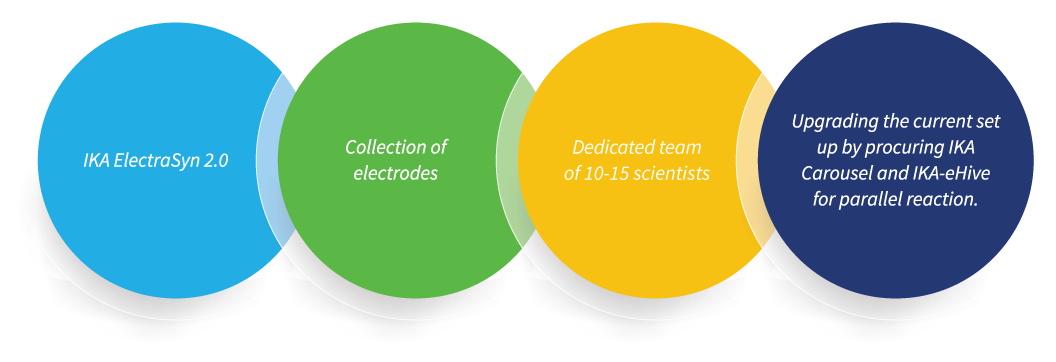
Jubilant Biosys is committed to this cause and has a dedicated team of scientists exploring novel methods to cater to the needs of our clients, and the recent launch of ElectraSyn 2.0 by IKA has helped us in this endeavour.
Our Capabilities
PhotoRedox Chemistry
After the first report of the PhotoRedox reaction by Prof. David MacMillan in Science 2008, there has been exponential growth in this field. A search in Reaxys with the code word “PhotoRedox Reaction” showed nearly 900 publications in 2021. In the last ten years, PhotoRedox catalysis has moved from academic research labs to industrial curiosity. According to Prof. MacMillan, every pharmaceutical company he visited is now doing PhotoRedox in medicinal chemistry, and thousands of drug-like compounds are being generated this way.
Many catalytic conversions like C-C bond forming (sp3-sp2 and sp3-sp3 cross-coupling), cycloaddition, challenging functional group conversion, and unusual rearrangement, which were either not feasible through conventional methods or lengthy routes are achieved using PhotoRedox reaction.

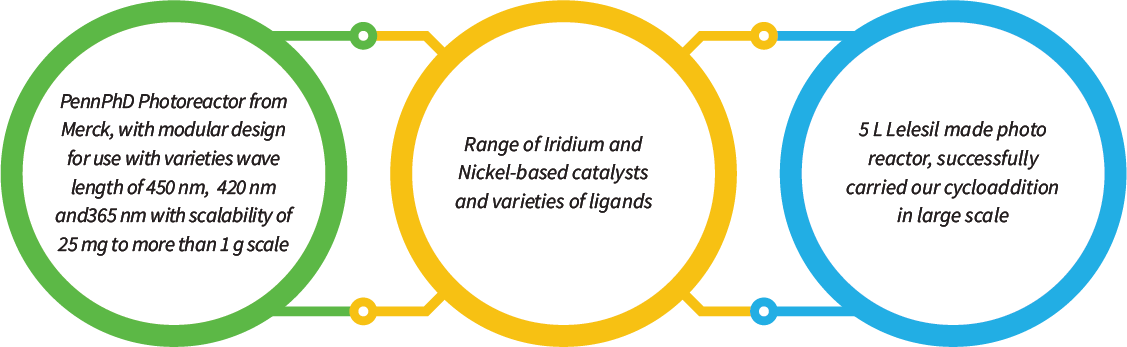
Jubilant Biosys is actively working on this technology by using a Penn PhD Photoreactor with a dedicated team of 10 scientists who are well-trained and efficient in successfully screening reaction conditions carried out several C-C bond formation reactions as well as functional group transformations. Jubilant has been supporting many of its collaborators through this new technology to test challenging and novel reaction conversion. We are now focusing on continuous-flow photochemistry by combining our flow reactor with a photo reactor, which will have excellent potential for scalability
Our Capabilities Comprise of
With our uniquely designed, digitally enabled state-of-the-art facility at CIRC, our Centers, of Excellence Support you in the drug discovery process.
